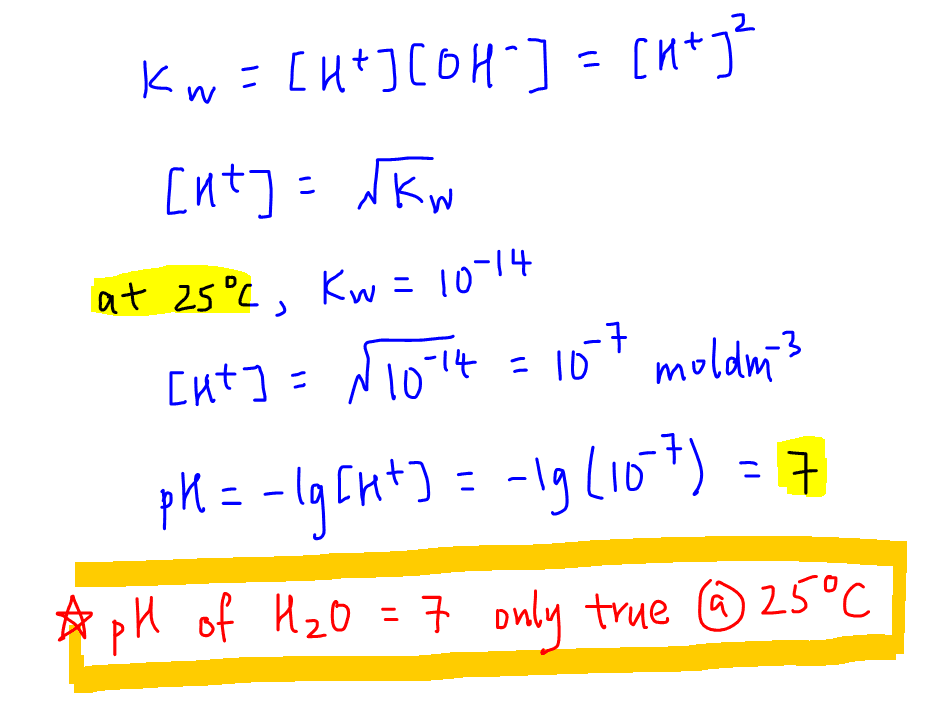The pH of water 40^0C is 6.8. If the density of water 40^0C is assumed to 1, g, cm^{-3}, its degree of dissociation will be10^{-13.6}/55.5655.56/ 10^{-13.6}55.56/ 10^{-6.5}10^{-6.8}/55.56

Unit 13 Acids and Bases. D. Finding the pH of Solutions Self- ionization of water – the simple dissociation of water H 2 O H + + OH - Concentration of. - ppt download
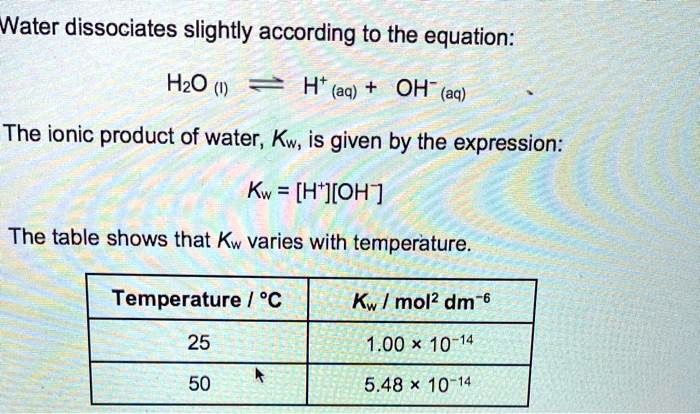
SOLVED: Calculate the pH of pure water at 50 degrees Celsius. Provide the answer to 2 decimal places and show your working. Water dissociates slightly according to the equation: H2O (l) ->










:max_bytes(150000):strip_icc()/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)