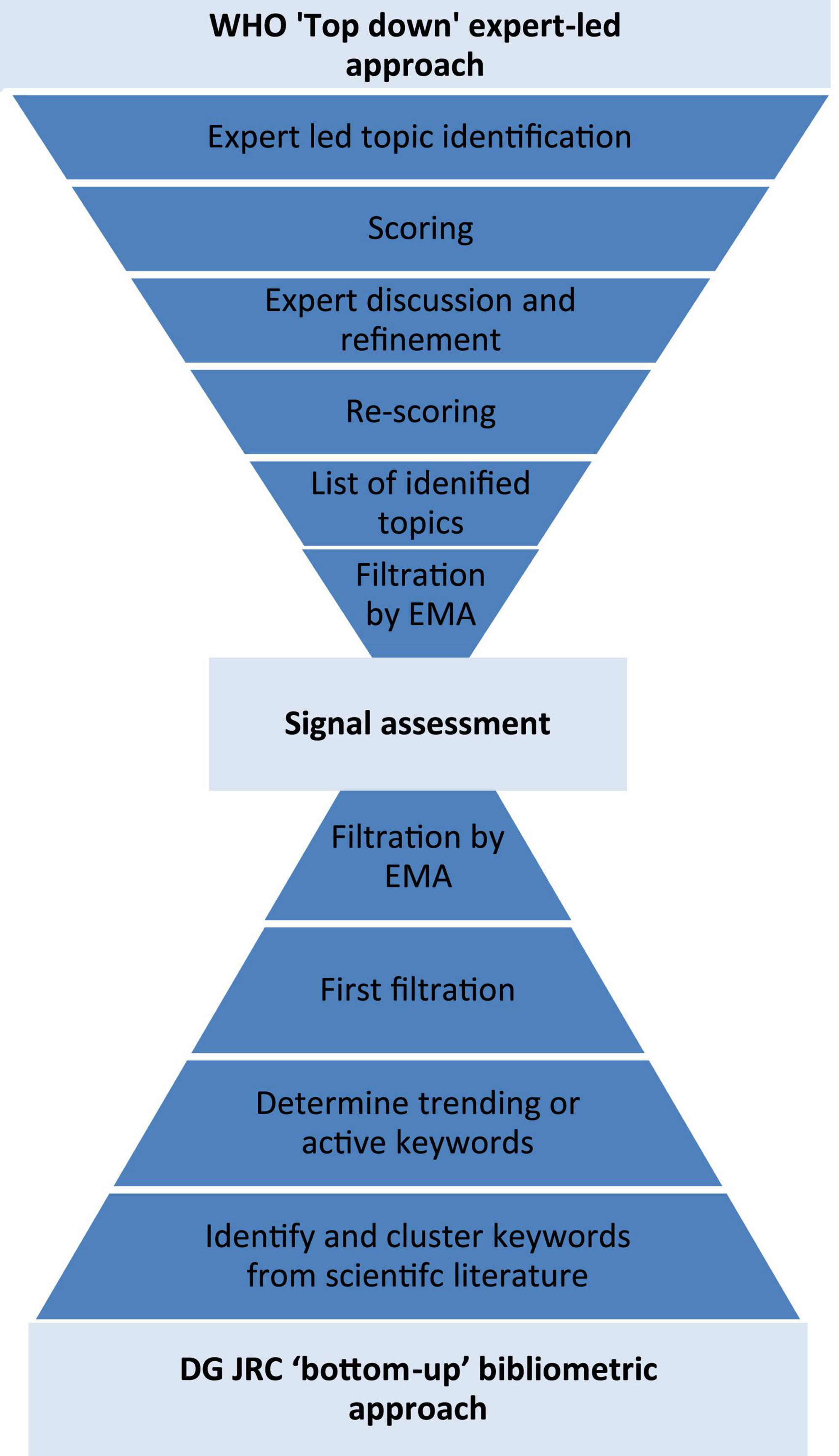
Frontiers | Health horizons: Future trends and technologies from the European Medicines Agency's horizon scanning collaborations

PDF) Does additional monitoring status increase the reporting of adverse drug reaction s ? An interrupted time series analysis of EudraVigilance data

Risk-Based Biologics: CMC Flexibilities in the EU Regulatory System - BioProcess InternationalBioProcess International
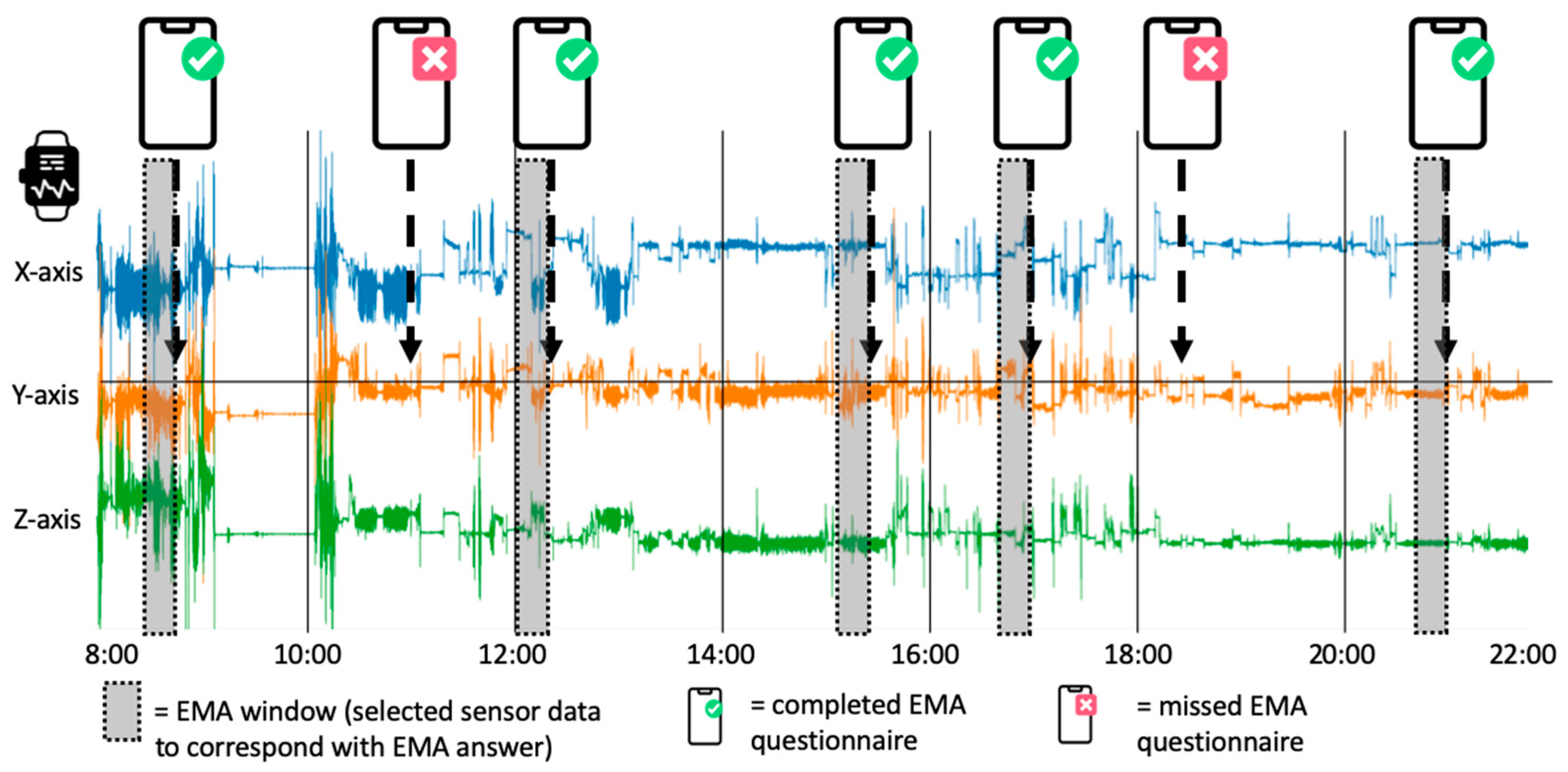
Data | Free Full-Text | A Long-Term, Real-Life Parkinson Monitoring Database Combining Unscripted Objective and Subjective Recordings

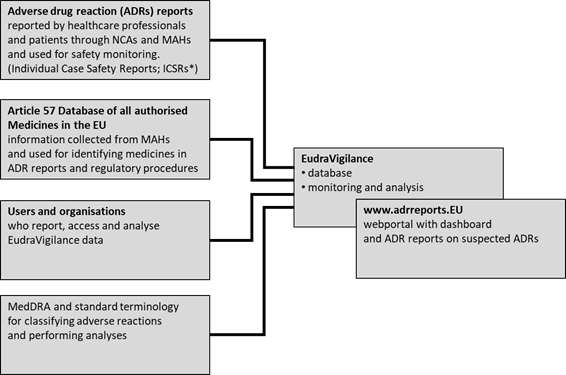
Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works. - Abstract - Europe PMC

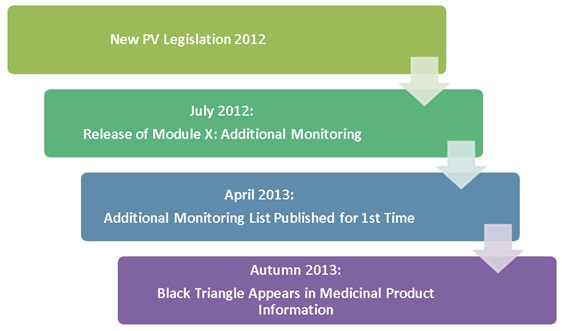
EU Commission Report on National and EMA experience on Medicines subject to Additional Monitoring released today

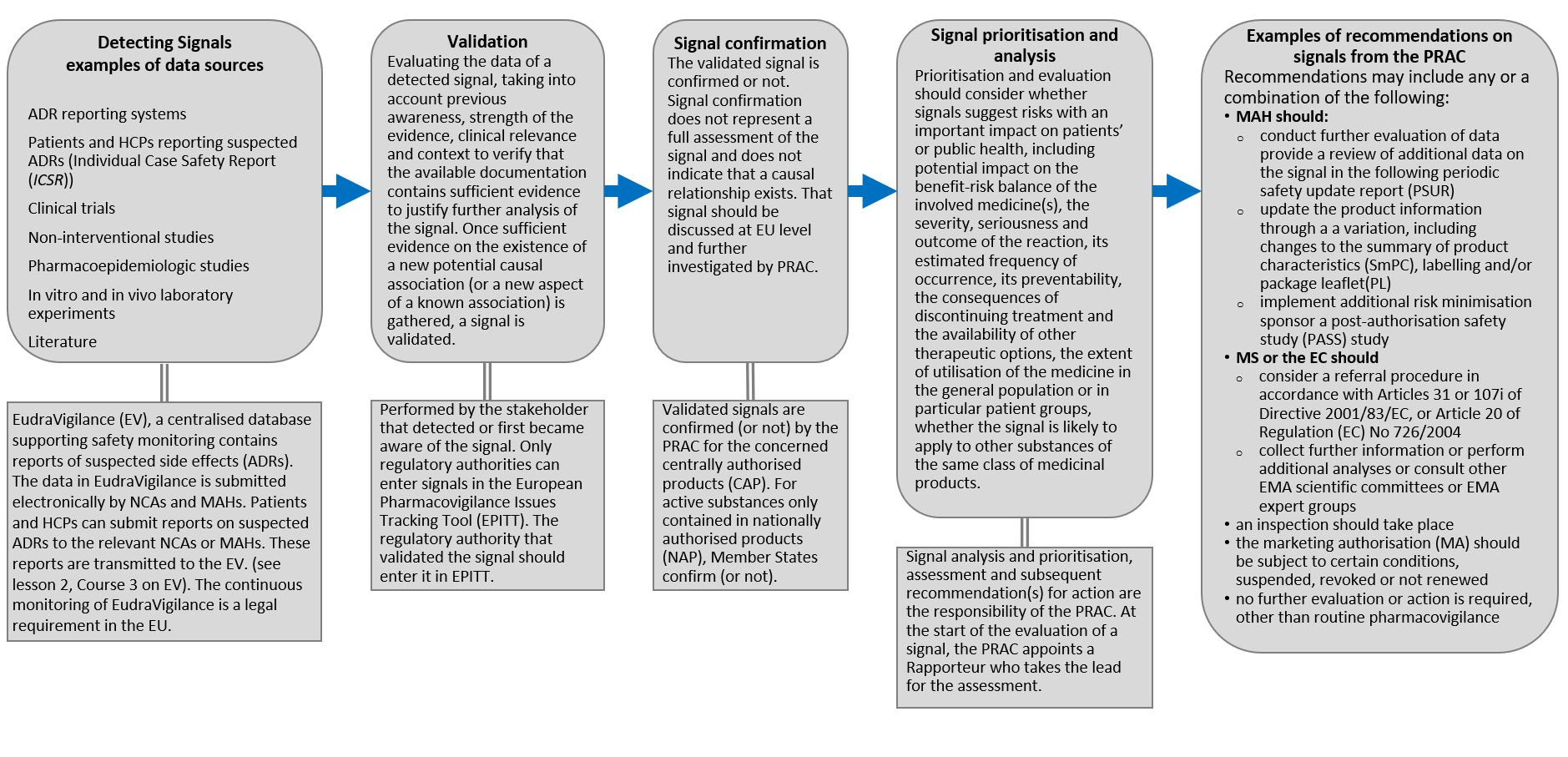
Improving the Safety of Medicines in the European Union: From Signals to Action - Potts - 2020 - Clinical Pharmacology & Therapeutics - Wiley Online Library